X-ray Fluorescence Spectroscopy (XRF)
|
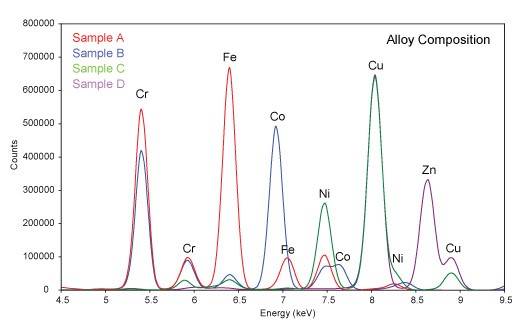
X-ray Fluorescence Spectroscopy (XRF) is a nondestructive technique for the chemical analysis of materials by measurement of the fluorescent x-rays produced when a sample is exposed to a source of short wavelength x-rays. X-ray from the source promote electrons in atoms of the sample into higher energy orbitals. When those electrons drop back to lower energy orbitals, they emit x-rays with wavelengths corresponding to the energy level differences of the orbitals. The energy level differences depend on the atomic number of the element, and the wavelength of the fluorescent x-rays are thus characteristic of the elements present. Since light elements produce x-rays with longer wavelengths which are difficult to detect, the XRF technique is generally limited to elements with atomic numbers of 13 (Al) or greater. Typical applications include elemental analysis of most solids, including minerals, inorganic materials, ceramics, metal alloys, and organometallic materials. XRF can also be used to determine heavy element impurities in polymers, plastics, and liquid solutions. XRF Instrumentation: Rigaku RIX 3001 Specifications: Radiation: Rh target, 3 kW Element range: Al - U Sample size: 20-50 mm disk Simultaneous detection of up to 50 elements Rigaku RIX 3001 X-ray Fluorescence Spectrometer Typical XRD Scan
|
Some of the links on this page may require additional software to view.